Why Group 1 Elements Are Called Alkali Metals
These elements will react vigorously and depending on which group 1 element youre dealing with even explosively with. The group 1 of the periodic table contain six elements namely Lithium Li Sodium NaPotassium KRubidium RbCesium Cs and Francium FrThese metals are called alkali metals because they form alkalies ie.

Chemistry Group 1 2 Knowledge Fountain Tutoring Facebook
Subsequently one may also ask why are the elements in Group 1 called alkali metals.

. 1 Answer anor277 Jun 14 2017. Solutions when they react with water which is why they are called alkali metals. CISCE ICSE Class 9.
Group-1 Elements of the Modern Periodic Table consist of Hydrogen H Lithium Li Sodium Na Potassium K Rubidium Rb Caesium Cs and Francium Fr. It is because their hydroxides are soluble bases called alkalies. Group I elements form strong hydroxides on reaction with water which are strong alkaline in nature.
I Highest melting point. The group 1 of the periodic table contain six elements namely LithiumLi SodiumNaPotassiumKRubidiumRbCesiumCs and FranciumFr. Strong bases capable of neutralizing acids when they react with water.
Group 1 I A elements are called alkali metals. 0 followers 0. Lithium Li Sodium Na Potassium K Rubidium Rb Cesium Cs and Francium Fr.
Secondly their ashes are alkaline in nature. The elements of the 1st GROUP of the periodic table are called alkali metals. Alkali metals are soft metals whereas.
Give a Reason for the Following. Science Anatomy Physiology Astronomy. Elements of the I A group are metals react with water to.
Among all the elements of the periodic table the alkali metals are considered to be the most electropositive. Caesium is known to be the most electropositive stable element. From the top to the bottom of the group the reactivity of alkali metals increases so lithium Li is the least reactive alkali metal and the most reactive is.
Group 1 elements form alkaline. These metals are called alkali metals because they form alkalies ie. Which element is most electropositive in group first.
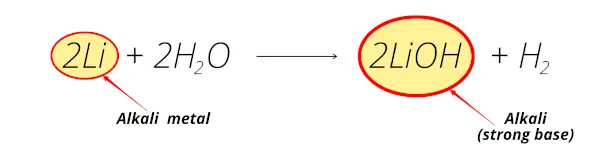
Group 1 elements are called alkali metals because their oxides are soluble in water and group 2 elements are called alkaline earth metals as their oxides form in the earth and are water soluble. They must be stored under oil to keep air and water away from them. 2Ms 2H2Ol 2MOHaq H2 g.
Alkali metals are also known to react violently and explosively with water. Posted by Marlene Dixon. Among the alkali metals which has.
Why 1st group element are called alkalines. Why are group 1 elements called alkali metals. Why are the elements of group IA called alkali metals.
They are called alkali metals because they form alkaline solutions when they react with water. Group 1 elements are indeed called alkali metals because of what happens when they react with water. Ii Most electropositive character.
Since they are unstable and they react rapidly to other elements they are never found uncombined in nature. Out of these elements except for hydrogen the remaining elements are popularly known as. And of course we commonly find the hydroxides of the alkali metals from extracting the ashes of a fire with water - hence potash ie.
Home Community Why do we say that more than one oxidation number for alkali metals are ruled out. Solve any question of The S-Block Elements with-. Which is the most electropositive element in Group 17 and why.
What did she mean by oxides. The elements of group 1 are called alkali metals because their oxides and hydroxides form alkaline solutions on treating with water. Strong bases capable of neutralizing acids when.
Group 1 elements are called alkali metals because of their ability to displace H2g from water and create a basic solution. With the exception of noble gases they bond well with all elements. This is because enough heat is given off during the exothermic reaction to ignite the H2g.
Why are group I elements called alkali metal. Group 1 I a Elements Are Called Alkali Metals. Advertisement Remove all ads.
The group 1 of the periodic table contain six elements namely Lithium Li Sodium NaPotassium KRubidium RbCesium Cs and Francium FrThese metals are called. The elements of the 17th GROUP of the periodic table are called halogens. So Iodine is the most electropositive element among.
The general form for the reaction of a group 1 element M with water looks like this. So group 1 elements are called alkali metals. Asked Dec 11 2020 in Chemistry by Maisa 457k points s-block elements.
The group 1 of the periodic table contain six elements namely LithiumLi SodiumNaPotassiumKRubidiumRbCesiumCs and FranciumFr. Click to see full answer. Therefore the solution becomes basic or alkaline due to the formation of hyroxide ions.
Downvote Inorganic chemistry Oxidation Alkali metals Chemistry Metals. In the periodic table alkali metals are the first category. Strong bases capable of neutralizing acids when they react with water.
These metals are called alkali metals because they form alkalies ie.

Meet The Element Families Of The Periodic Table Alkaline Earth Metals Periodic Table Alkali Metal

Pin On Class 11 Chemistry Notes

Comparing Halogen Reactivity Trends With Those Of The Alkali Metals Halogens Become Less Reactive Down The Chemistry Classroom High School Chemistry Chemistry

Meet The Element Families Of The Periodic Table Alkaline Earth Metals Periodic Table Alkali Metal
Why Group 1 Elements Are Also Called Alkali Metals Homeworklib

Pin By Anushree Roy On School Chemistry 11th Chemistry Chemistry Notes
Why Are Group 1 Elements Called Alkali Metals

Alkali Metals By Manofgamesyt Issuu

Chem Guide Alkali Metals Chemistry Ochem Organic Chemistry

Alkali Metal Definition Properties Facts Britannica

The Elements Of Group 1 Are Called Alkali Metals Because

Why S Block Elements Are Called Alkali Metals

Chemistry Group 1 2 Knowledge Fountain Tutoring Facebook

The S Block Elements Cbse Notes For Class 11 Chemistry Learn Cbse 11thchemistrynotes Thes Blockeleme Chemistry Notes 11th Chemistry Notes 11th Chemistry

Alkali Metals Elements In Group 1 Are Called Alkali Metals Ppt Download

Trick To Memorize Group 2 Elements Of Periodic Table

Alkali Metals Are In Group I Of The Periodic Table Alkali Metal Picture Folder Interactive Multimedia

Elements In Groups 1a 2a And 3a 8a Are Known As The Main Group Elements Main Group Elements Are In The S Block And The P Block The Electron Configurations Ppt Download

Comments
Post a Comment